The tumor biomarker research team from Suzhou Institute of Biomedical Engineering and Technology of Chinese Academy of Sciences recently reported a key role of DNA methylation-deregulated and RNA m6A reader-cooperating lncRNA (DMDRMR) in tumor initiation and progression, potentially providing a diagnostic, prognostic and therapeutic target for treating clear cell renal cell carcinoma (ccRCC) patients.
Cancer is a major global health issue. To improve survival of cancer patients, it is urgent for researchers to identify potential biomarkers for early diagnosis and therapeutic targets. With the tumor- and tissue-specificity, DNA methylation and long non-coding RNA (lncRNA) play critical roles in tumor initiation and progression, making them ideal targets for tumor diagnosis and treatment.
In this research, scientists utilized data from 12 cancer types from The Cancer Genome Atlas to comprehensively map lncRNA that are potentially deregulated by DNA methylation, and identified that DMDRMR is a higher expression by its hypomethylated promoter in tumors compared to adjacent normal tissues. Both lower methylation and higher expression level of DMDRMR are associated with poor outcomes for ccRCC patients. Furthermore, DMDRMR binds to insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) to stabilize target genes, including the cyclin-dependent kinase 4 (CDK4) and three extracellular matrix components (fibronectin 1 (FN1), collagen type VI alpha 1 chain (COL6A1), and laminin subunit alpha 5 (LAMA5)), by specifically enhancing IGF2BP3’s reading on them in an m6A-dependent manner. The complex of DMDRMR/IGF2BP3 binds to 5' untranslated region (UTR) of CDK4 transcript, which is modified by m6A, and increases CDK4 expression. Consequently, the G1/S transition and cell proliferation are accelerated, and the resistance to CDK4/6 inhibitor palbociclib is enhanced. In addition, the complex of DMDRMR/IGF2BP3 binds to the exon 20 of FN1 transcript, which is modified by m6A, and increases FN1 expression. Consequently, the metastasis and invasion of ccRCC cell lines are enhanced. These results demonstrated that DMDRMR acts as a cofactor for IGF2BP3 to stabilize target genes in an m6A-dependent manner, thus playing an essential oncogenic role in ccRCC.
They also found that DMDRMR/IGF2BP3 axis shows high expression and positive association with ccRCC patients, and a high coexpression of DMRRMR and IGF2BP3 is associated with poor outcomes for ccRCC patients. In summary, this study provides a potential therapeutic target and its potential clinical application in ccRCC treatment.
This research is supported by the National Natural Science Foundation of China, and the research article "DMDRMR-mediated regulation of m6A-modified CDK4 by m6A reader IGF2BP3 drives ccRCC progression" has been published in Cancer Research.
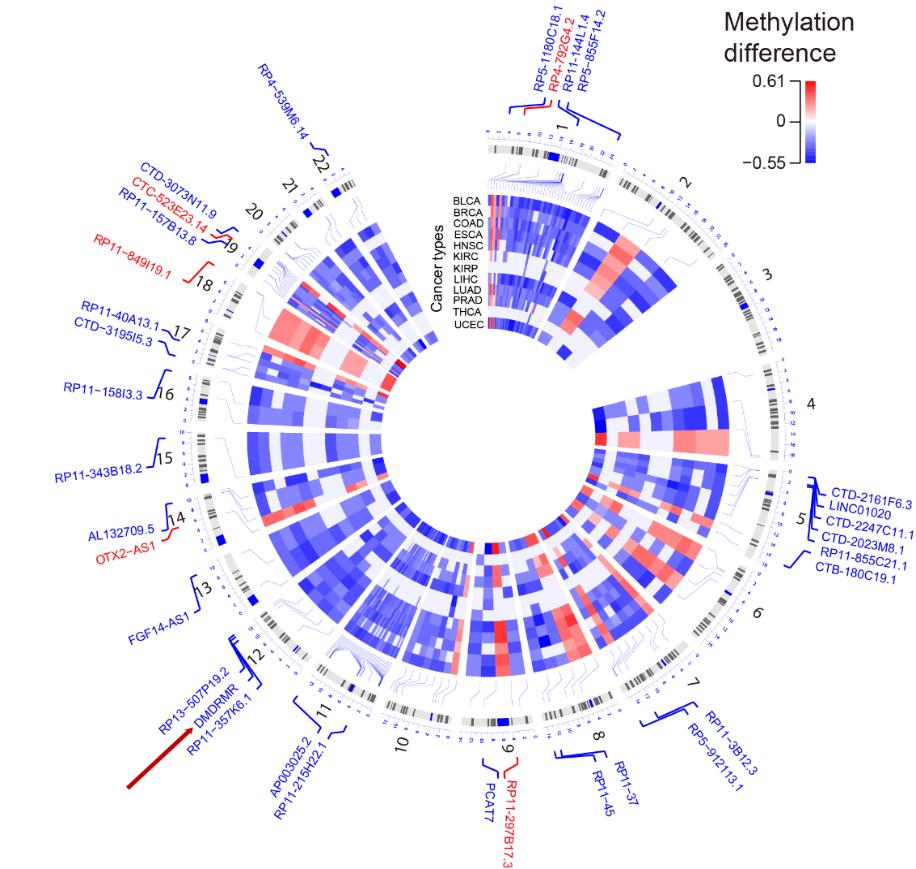
Figure 1. Circos plot of 28 common hypomethylated (blue) and 5 common hypermethylated (red) promoters identified across 12 cancer types. (Image by SIBET)
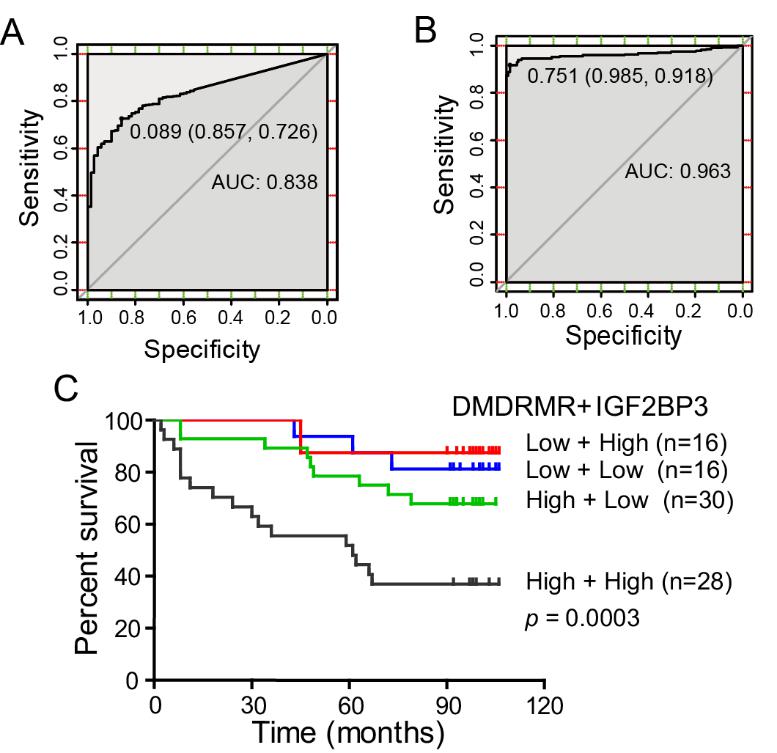
Figure 2. DMDRMR is a potential diagnostic and prognostic target for ccRCC. The ROC curves are based on DMDRMR expression (A) and promoter methylation (B) levels for discrimination of ccRCC tissues. (C) Kaplan–Meier analysis indicating the correlation of the combination of cytoplasmic DMDRMR and IGF2BP3 expression with percentages of survived ccRCC patients. (Image by SIBET)
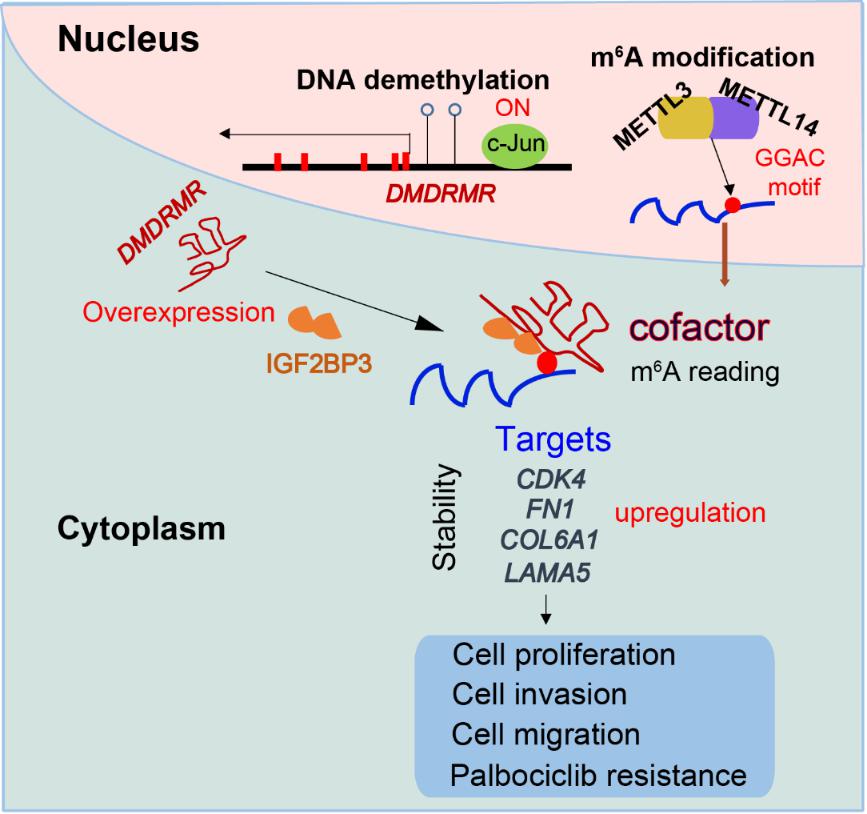
Figure 3. Proposed model for the DMDRMR/IGF2BP3 axis promoting the pathogenesis of ccRCC. (Image by SIBET)
Contact
XIAO Xintong
Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences (http://www.sibet.cas.cn/)
Phone: 86-512-69588013
E-mail: xiaoxt@sibet.ac.cn