Recently, researchers from Suzhou Institute of Biomedical Engineering and Technology (SIBET) and South China University of Technology collaborated to develop a near-infrared (NIR)-driven nanoassemblies with size and charge dual transformation for the combination of photocontrolled chemotherapy and immunotherapy in breast cancer.
Nanotechnology has unique advantages in improving the bioavailability of poorly soluble substances, achieving controllable and targeted drug release, and integrating different therapeutic modalities in the same platform.
However, it faces various biological barriers for practical applications, including blood circulation, trans vascular transport, malformed tumor vessels, and dense tumor extracellular matrix, resulting in most of nanoparticles are mainly localized around the peripheral of the tumor, and it is difficult to penetrate into the tumor to exert cell killing effect.
In their work, researchers designed diselenide-bridged mesoporous organosilica nanoparticles (MONs) as a reactive oxygen species (ROS)-responsive core for the chemotherapeutic agent doxorubicin (DOX) loading.
They then coated an indocyanine green (ICG)-hybrid N-isopropyl acrylamide (NIPAM) layer to form a thermosensitive shell.
"The negatively charged thermosensitive layer prevents DOX leakage, rendering prolonged blood circulation time and high tumor accumulation,” said DONG Wenfei, one of the researchers from SIBET.
Upon NIR light irradiation, mild photothermal effects facilitate the dissociation of the thermosensitive shell to achieve negative-to-positive surface charge reversal. Meanwhile, ICG-generated ROS cleave the diselenide bond of the organosilica core, resulting in rapid matrix degradation that produces DOX-containing smaller fragments (115nm to 20nm).
Such an NIR light-driven charge and size dual-transformable nanoassemblies facilitate tumor accumulation and deep penetration, amplify chemotherapy potency, and evoke robust immunogenic cell death effects in vitro and in vivo.
In animal studies, with the combination of a programmed cell death protein-1 (PD-1) checkpoint blockade, the nanosystem remarkably blocks primary tumor growth and pulmonary metastasis of breast cancer with greatly alleviating the toxic and side effects of free drugs.
This work provides a new platform for the safe and efficient combination therapy of breast cancer. The team will modify antibody on the surface of the nanoassembly to enhance the active targeting of tumors, and try to use this system to carry gene editing tools for gene therapy of tumors.
The results have been published in the journal Theranostics entitled “A light-driven dual-nanotransformer with deep tumor penetration for efficient chemo-immunotherapy”.

Figure 1. Research results become journal covers. (Image by Theranostics)
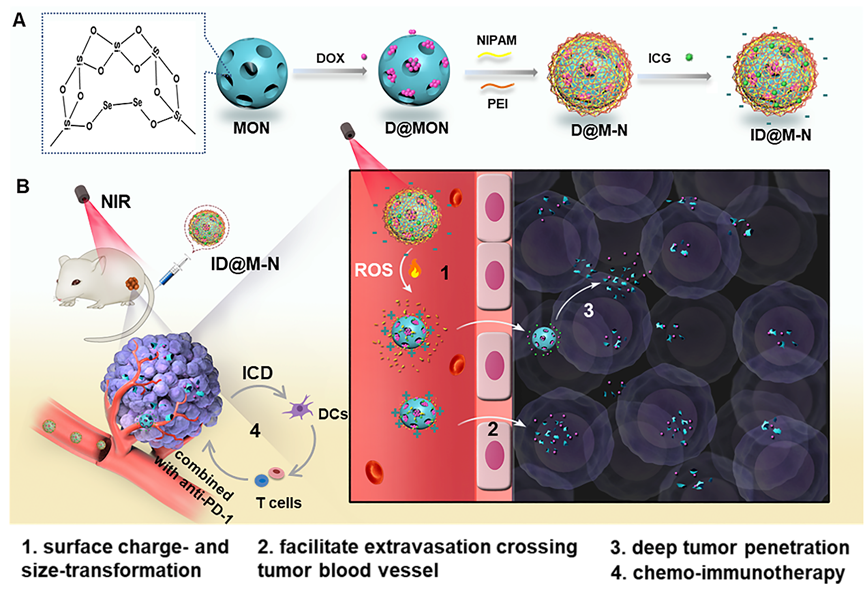
Figure 2. Nanoassemblies (ID@M-N) for NIR-triggered chemo-immunotherapy. (Image by SIBET)
Contact
XIAO Xintong
Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences (http://www.sibet.cas.cn/)
Phone: 86-512-69588013
E-mail: xiaoxt@sibet.ac.cn