The tumor biomarker research team from the Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, recently reported that DMDRMR/miR-378a-5p/ DAB2IP axis promotes angiogenesis and sunitinib resistance, potentially providing a diagnostic, prognostic and therapeutic target for treating clear cell renal cell carcinoma (ccRCC) patients. These findings further expanded and highlighted their previous research "DMDRMR-mediated regulation of m6A-modified CDK4 by m6A reader IGF2BP3 drives ccRCC progression", which was published in Cancer Research on Feb. 2021.
ccRCC is a major type of RCCs and characterized by being highly angiogenic and densely vascularized. Some important genes are frequently mutated in RCC, including Hippel-Lindau (VHL) tumor suppressant gene, resulting in abnormal activation of downstream proteins and signaling pathways, and leading to neovascularization, thereby promoting the tumorigenesis, development and metastasis of this tumor. Thus, targeted angiogenesis inhibitors, such as sunitinib and sorafenib, have been used as first-line therapy for patients with advanced RCC. However, most patients develop acquired drug resistance to the angiogenesis therapy. Therefore, further understanding of tumor angiogenesis for ccRCC is highly desired.
Vascular endothelial cell growth factor A (VEGFA), a member of the VEGF family, is the strongest vascular growth factor in inducing tumor angiogenesis. Tumor cells generally are highly expressed for VEGFA, which promotes endothelial cell differentiation, proliferation, migration, and angiogenesis by binding to its primary receptor VEGFR2. Thus, VEGFA/ VEGFR2 signaling pathways play a key role in tumor angiogenesis. Last year, they showed that a long non-coding RNA, DNA methylation-deregulated and RNA m6A reader-cooperating lncRNA (DMDRMR) acts as a cofactor for insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) to stabilize target genes in an m6A-dependent manner, thus exerting essential oncogenic roles in ccRCC. Additionally, they also found that DMDRMR increases the expression level of VEGFA and regulates the biological pathways associated with angiogenesis, but these effects are independent of its binding protein IGF2BP3. Based on this, a new scientific question has been raised: whether and how does DMDRMR promote neovascularization in ccRCC?
In this study, they demonstrated that DMDRMR serves as a sponge of miR-378a-5p to increase enhancer of zeste homologue 2 (EZH2) and smad ubiquitination regulatory factor 1 (SMURF1) expression, thus promoting EZH2-mediated transcriptional repression of DOC-2/DAB2 interactive protein (DAB2IP) and SMURF1-mediated degradation of DAB2IP. Consequently, this axis activates VEGFA/VEGFR2 signaling pathway and promotes angiogenesis and resistance of tumor cells to sunitinib in ccRCC. They also found that DMDRMR/miR-378a-5p/IGF2BP3 axis shows high expression and positive association with ccRCC patients, and the combination of overexpressed DMDRMR and downregulated DAB2IP predicted the poorest overall survival of ccRCC patients, supporting that the axis may serve as a novel target for combination diagnosis/therapy of ccRCC patients. These findings may have high clinical relevance for future translation to develop targeted therapies for patients with ccRCC.
This research is supported by the National Natural Science Foundation of China and the Strategic Leadership program of the Chinese Academy of Sciences, and the research article "DMDRMRPromotes Angiogenesis via Antagonizing DAB2IP in clear cell Renal Cell Carcinoma" has been published in Cell Death and Disease. ZHU Yumeng and LIU Xiaojun are the first authors of this paper, and GAO Shan and GU Yinmin are the co-corresponding authors. The tumor biomarker research team led by Prof. GAO has made a series of achievements in the identification of tumor biomarkers, and has published a number of peer-reviewed research papers, including Proceedings of the National Academy of Sciences (2), Science Advances, and Cancer Research (2), etc.
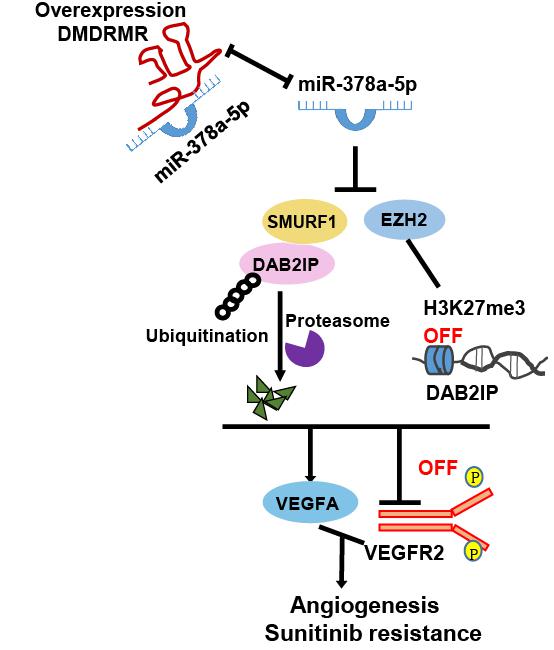
Figure 1. Proposed model for the DMDRMR/miR-378a-5p/DAB2IP axis promoting the angiogenesis and sunitinib resistance of ccRCC. (Image by SIBET)
Contact
XIAO Xintong
Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences (http://www.sibet.cas.cn/)
Phone: 86-512-69588013
E-mail: xiaoxt@sibet.ac.cn