Recently, a research team led by Professor MIAO Peng at the Suzhou Institute of Biomedical Engineering and Technology proposed a nano-impact electrochemical (NIE) sensing strategy for highly sensitive determination of miRNA.
The new mechanism and results were published in recent issue of Nano Letters entitled "Nano-Impact Electrochemical Biosensing Based on a CRISPR-Responsive DNA Hydrogel".
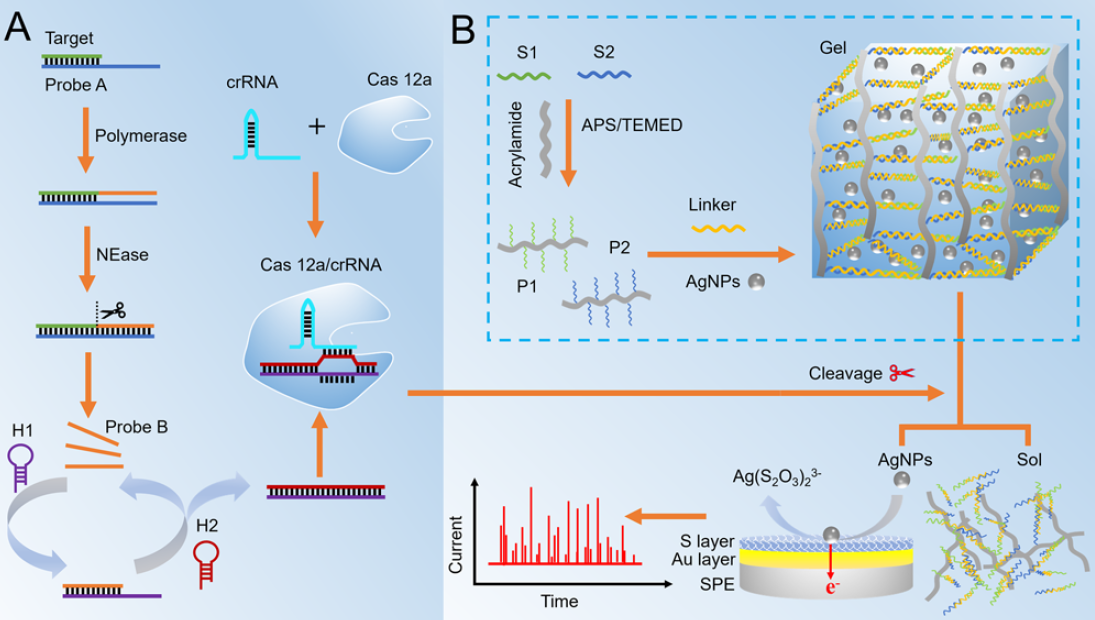
Fig. 1 (A) Illustration of target-induced SDA and CHA. (B) Illustration of DNA hydrogel formation and CRISPR/Cas-digested event for the electrochemical response of released AgNPs. (Image by SIBET)
NIE allows the examination of the physicochemical properties and structure-activity relationships of nanomaterials at nanometer scale. It helps achieve biomolecule analysis in a simple, rapid, and high-throughput manner, however, the relatively low effective collision frequency limits its broad prospects in basic research and clinical application.
"We therefore proposed a novel NIE sensing strategy amplified by the CRISPR-responsive DNA hydrogel and cascade DNA assembly, significantly enhancing the sensitivity of this biosensor,” said MIAO Peng.
The entire process included target miRNA-induced strand displacement amplification (SDA), catalytic hairpin assembly (CHA), and CRISPR/Cas trans-cutting.
By loading nanomaterials into DNA hydrogels, the limitation of one target triggering one collision entity in traditional NIE mode could be overcome. In addition, the use of current frequency instead of peak current intensity mitigated the background interference, addressing the issue of poor stability in traditional electrochemical methods.
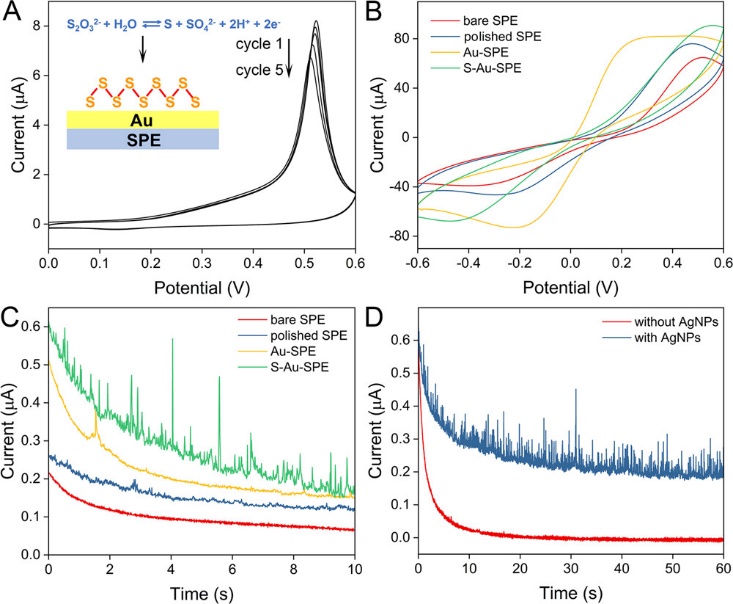
Fig. 2 (A) Cyclic voltammograms of Au-SPE in 10 mM Na2S2O3 and 10 mM NaOH. (B) Cyclic voltammograms for each modification step. (C) Current–time curves of AgNPs for different SPEs. (D) Current–time experiments with and without AgNPs. (Image by SIBET)
This biosensing strategy ensured ultra-high sensitivity, with a limit of detection for miR-141 as low as 4.21 aM. Subsequently, the researchers measured the reactions triggered by four mismatched miRNAs and further tested the application of this method challenging real samples. The method exhibited high selectivity and could successfully distinguish cells with abnormal miRNA levels.
This work has broad applicability in nucleic acid sensing, providing new insights into label-free and unmodified nano-electrochemical approaches, according to the researchers.
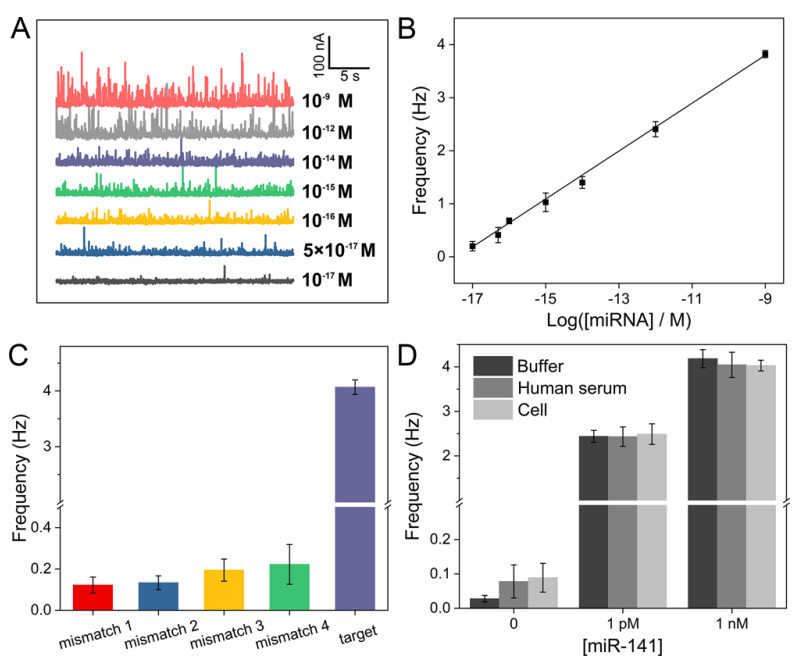
Fig. 3 (A) Current–time curves for the detection of miR-141. (B) Linear relationship between the collision frequency and the logarithm of miR-141 concentration. (C) Selectivity of the NIE method. (D) NIE method for the detection of miRNA spiked in standard buffer, human serum, and cell conditions. (Image by SIBET)
Doctoral student GUO Jiarong is the first author and Professor MIAO Peng is the corresponding author. This research is supported by the Natural Science Foundation for Distinguished Young Scholar of Jiangsu Province.
Contact
XIAO Xintong
Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences (http://www.sibet.cas.cn/)
Phone: 86-512-69588013
E-mail: xiaoxt@sibet.ac.cn