MicroRNAs (MiRNAs) are valuable biomarkers for the diagnosis and prognosis of diseases. The 2024 Nobel Prize in Physiology or Medicine has been awarded to American scientists Victor Ambros and Gary Ruvkun for their discovery of miRNAs which play important roles in post-transcriptional gene regulation.
Many miRNAs are closely related to the pathogenesis of tumors, including cell proliferation, invasion. The establishment of reliable miRNA diagnostic models can help in the early detection of a wide range of high-risk precancerous lesions.
Researchers at Suzhou Institute of Biomedical Engineering and Technology (SIBET) of the Chinese Academy of Sciences recently proposed a novel strategy based on DNA nanostructure disintegration-assisted strain-promoted alkyne–azide cycloaddition (SPAAC) ligation for highly sensitive detection miRNA (Figure 1).
The researchers, led by Professor MIAO Peng, designed a three-dimensional DNA triangular pyramid frustum (TPF) as the scaffold for target recognition, which not only retains a DNA triangle bottom with three thiol vertexes for firm immobilization at the gold surface but also provides three single-stranded lateral edges for subsequent DNA structural transitions.
In the presence of target miRNA, duplex-specific nuclease (DSN) is utilized to digest the single-stranded DNA at the top of the DNA TPF, which can lead to the recovery of a DNA triangle and then benefit the following SPAAC ligation to locate multiple signal strands.
SPAAC is a kind of click reaction without any metal and enzyme between azides and cyclooctyl alkynes, which has high selectivity and biological orthogonality. It is suitable for the ligation of discrete sites of DNA nanostructures.
Due to the design of lateral edges of the DNA TPF, multiple hairpins with ferrocenes can be formed, which shorten the distance between electrochemical species and the electrode (Figure 2).
By recording and analyzing the responses, researchers established a highly sensitive electrochemical biosensor, which exhibits high sensitivity and reproducibility. Clinical applications showed good stability (Figure 3).
This sensing strategy relies on the integration of DNA nanostructures and click chemistry, which may inspire further designs for the development of DNA nanotechnology and applications in clinical chemistry.
The research results entitled “DNA Nanostructure Disintegration-Assisted SPAAC Ligation for Electrochemical Biosensing” were published in Nano Letters.
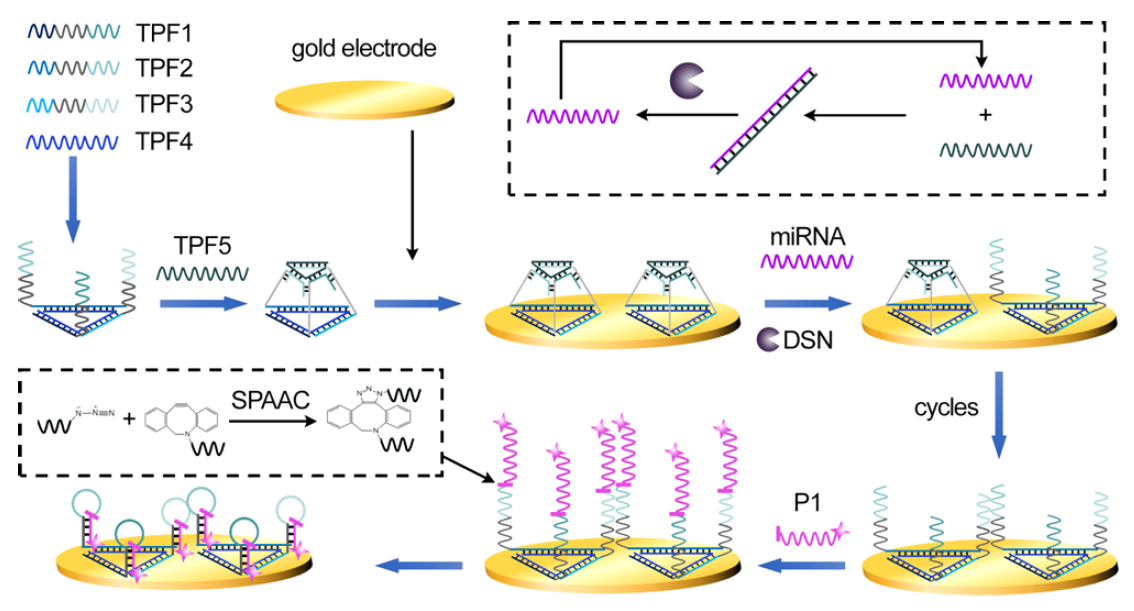
Figure 1. Illustration of DNA nanostructure disintegration-assisted SPAAC ligation and DSN signal amplification. (Image by SIBET)
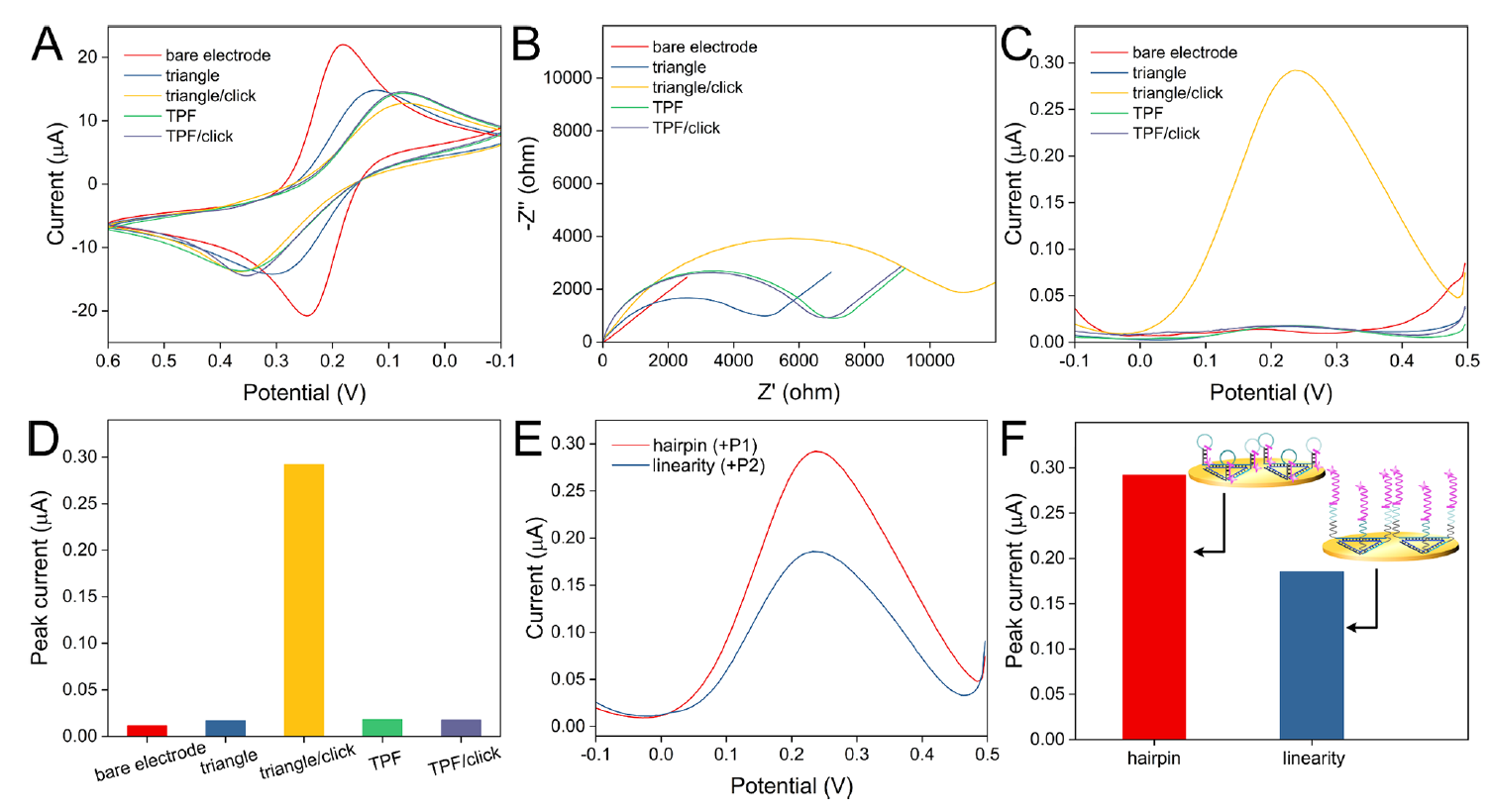
Figure 2. Electrochemical characterization of electrode modification and signal enhancement effect of hairpin structure. (Image by SIBET)
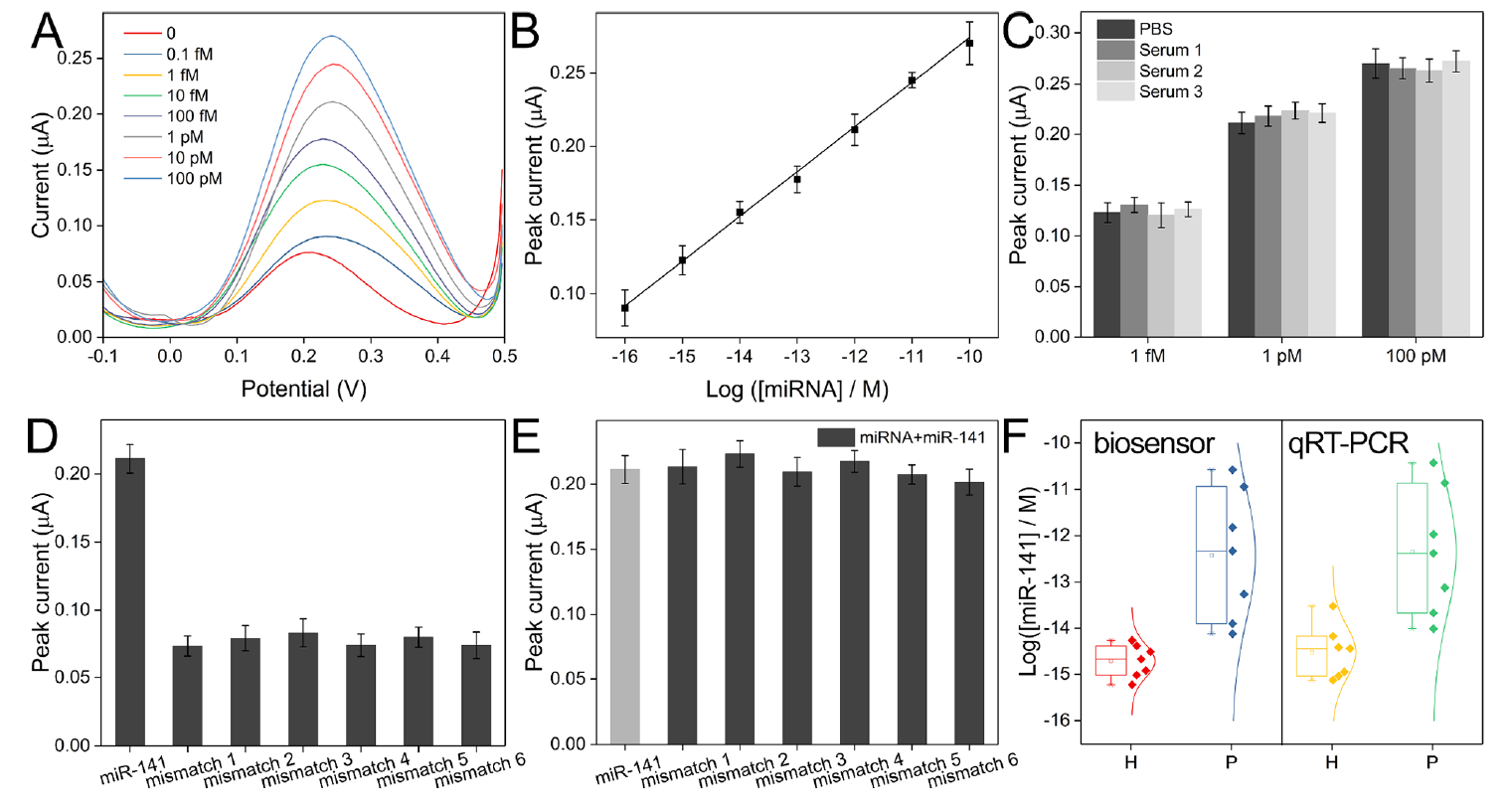
Figure 3. (A) Square wave voltammograms for the detection of miRNA with a series of concentrations. (B) Linear relationship between the logarithm of miRNA concentration and the peak current intensity. (C) Recorded peak currents for the analysis of miRNA of different concentrations spiked in standard buffer and serum samples. (D) Comparison of peak currents for the analysis of miR-141 and mismatched sequences. (E) Comparison of peak currents for the analysis of the mixtures of miR-141 and mismatched sequences. (F) Box plot of detected miR141 concentrations in samples from healthy individuals and patients. (Image by SIBET)
Contact
XIAO Xintong
Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences (http://www.sibet.cas.cn/)
Phone: 86-512-69588013
E-mail: xiaoxt@sibet.ac.cn