High throughput imaging and screening is essential for biomedical research and drug discovery using miniature model organisms such as Zebrafish.This study introduces a high-speed imaging system which illuminates Zebrafish embryos flowing through a capillary tube with a sheet of light and captures them using a linear charge-coupled device (CCD). This system can image dozens of zebrafish embryos per second. An image algorithm was developed to recognize each embryo and to perform automatic analysis. We distinguished dead and living embryos according to the gray level distribution and conducted statistics of morphological characteristics of embryos at different growing stages.
Our Linear CCD based flow imaging system (Lc-FIS) , which is in principle similar imaging cytometer but primarily for zebrafish embryos instead of cell. Lc-FIS can image up to 20 embryos per second which is hundred-times faster than previously reported VAST system. Furthermore, we developed image recognition algorithms to segment embryos with/without chorions. The system and algorithms were used to stud the cumulative mortality rate dependent and development asynchronization of zebrafish embryos. Our system and methods will satisfy the demanding for high throughput analysis in studies of developmental biology and drug discovery.
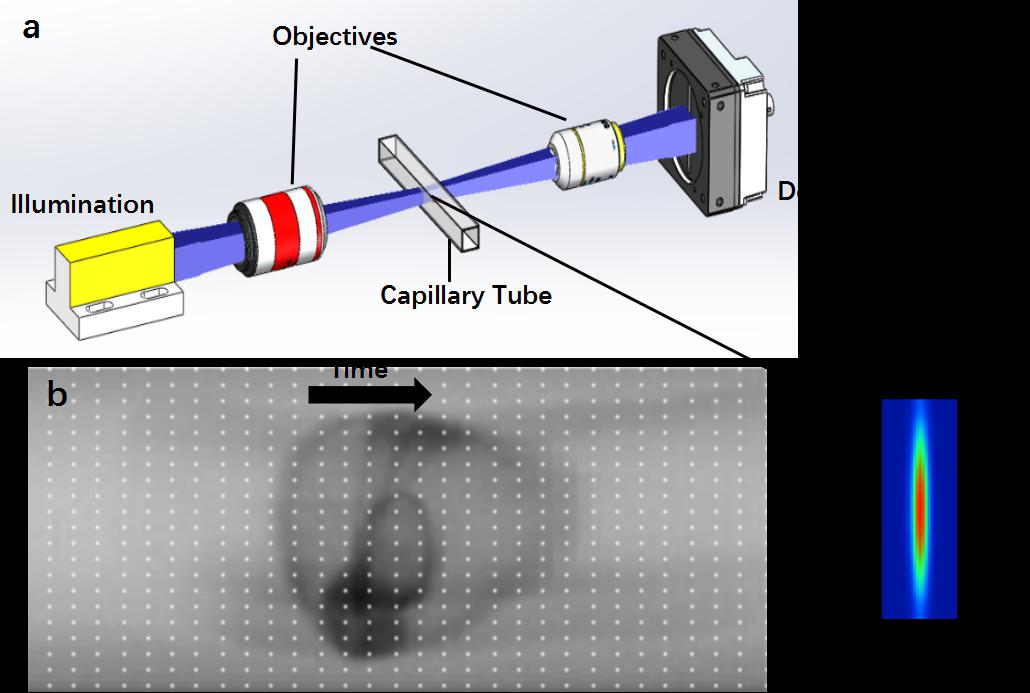
Fig. 1. Principle of Lc-FIS. (a) A sheet of light was focused by an objective to illuminate section of a capillary tube while embryos flowed through. The transmitted light signal was recorded by a fast linear-CCD camera at the other side of the tube. Inset shows the intensity profile of the light sheet at focus plane.(b)Arranging time series 1D linear CCD data into a 2D image.The black arrow indicates the flow direction.
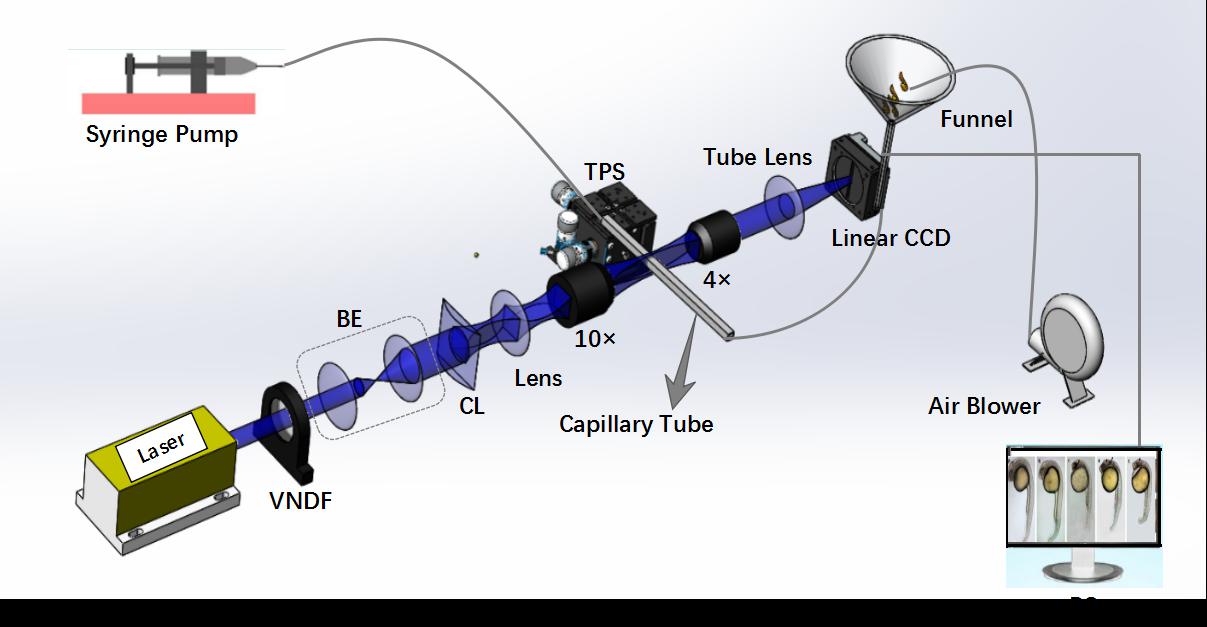
Fig. 2. Schematic illustration of Lc-FIS. In the fluidic part, samples were loaded to a funnel reservoir and driven through a capillary tube by a stable syringe pump. Air blower was used to prevent blocking. In the imaging part, a light sheet created by a cylindrical lens was focused on the center of capillary tube perpendicularly. Transmitted light signal was recorded by a linear CCD. VNDF, variable neutral density filter; BE, beam expander; CL, cylindrical lens; TPS, three-axis position stage.
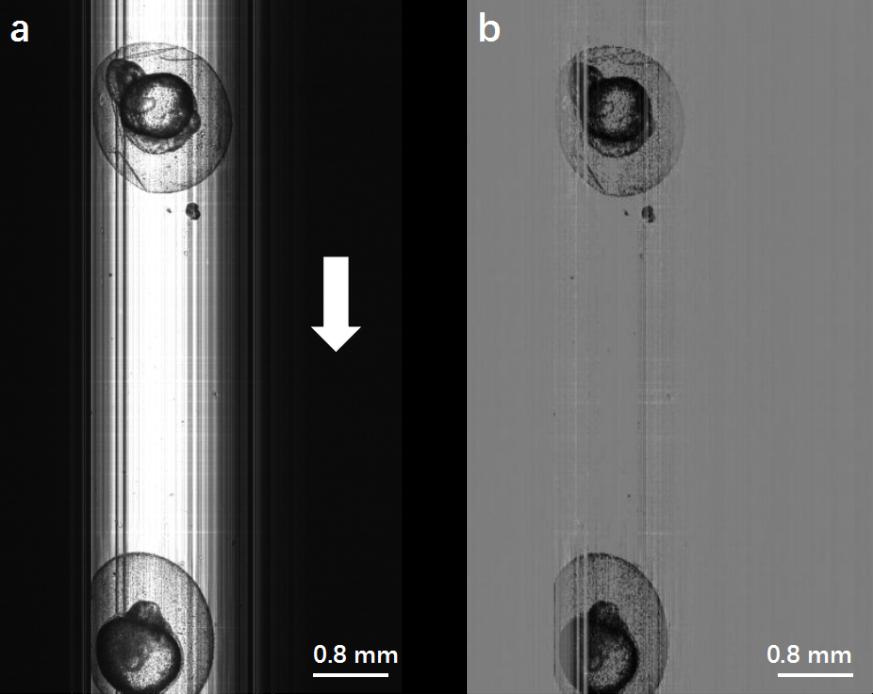
Fig. 3. (a) Original image of zebrafish embryos obtained by Lc-FIS. The white arrow indicates the flow direction. (b) Preprocessed image after substracting the background.
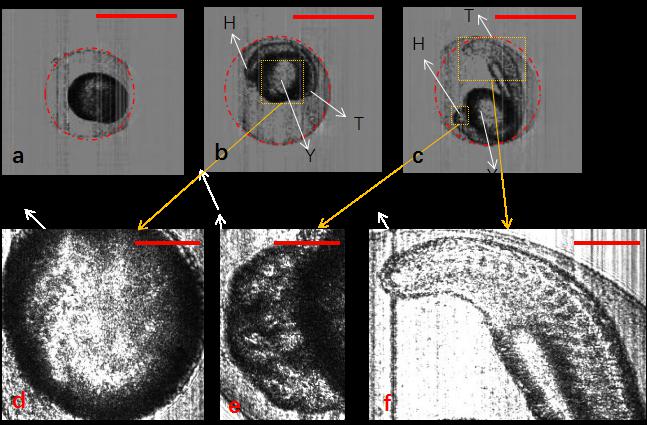
Fig. 4. Segmentation of zebrafish embryos with chorion using Hough-transformation circle detection algorithm. (a–c) Zebrafish embryos at different development stages: 3, 13, and 21.5 hpf, respectively. (d–f) Detailed structure of yolk, head, and tail of zebrafish embryos. H, head; T, tail; Y, yolk. The scale bars in (a)–(c) represent 1 mm, and those in (d)–(f) represent 0.3 mm.
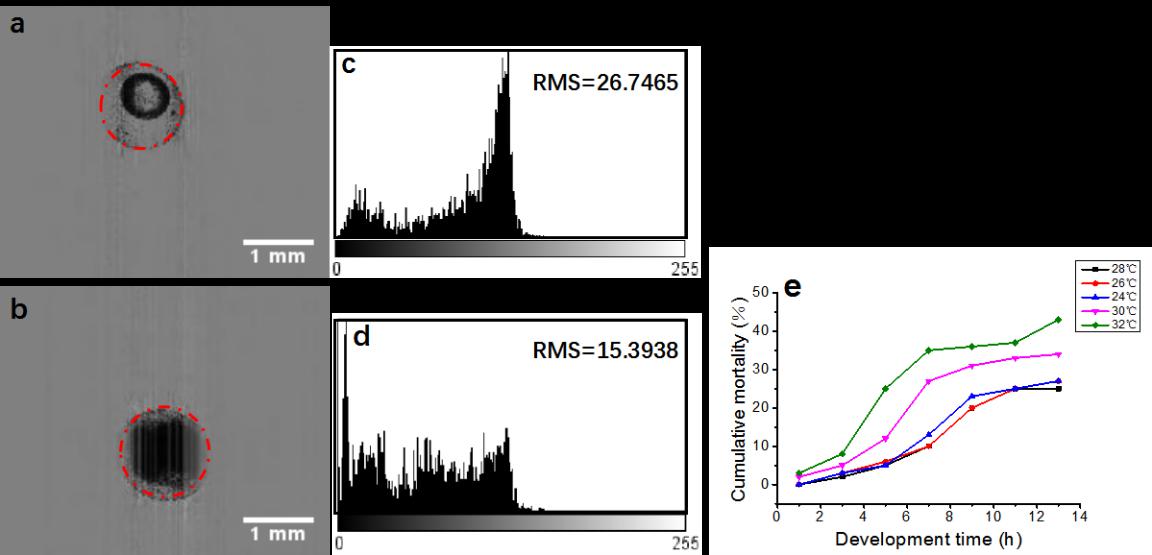
Fig. 5. Discrimination of live and dead embryos by Lc-FIS. (a) Normal and (b) dead embryos (4 hpf) detected using the Hough-transformation algorithm. (c) and (d) Intensity distributions within the circle of the live and dead embryos, respectively. (e) Cumulative mortality rate of zebrafish embryos at temperatures of 24°C, 26°C, 28°C, 30°C, and 32°C.
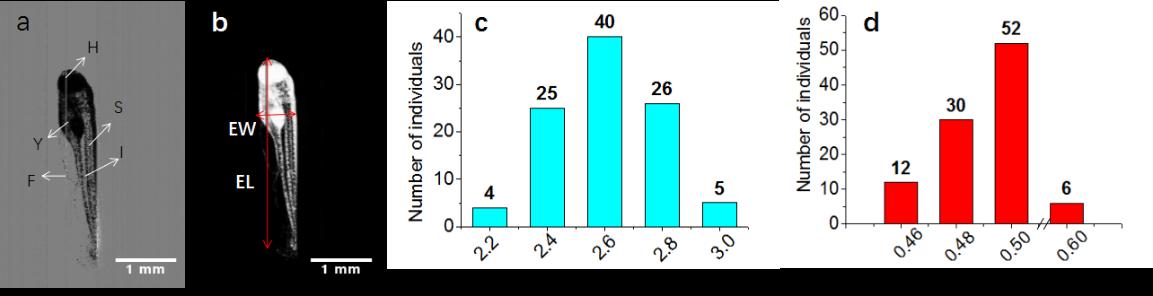
Fig. 6. Morphology statistics of live zebrafish embryos (72 hpf) without chorion. (a) Preprocessed image after subtracting background from the original image. (b) Binary image obtained after segmentation. The vertical and horizontal arrows indicate the EL and EW, respectively. (c) EL and (d) EW distributions of 100 zebrafish embryos (72 hpf). H, head; F, fin; I, intestine; S, somites; Y, yolk. The scale bars in (a) and (b) represent 1 mm .