Hepatocellular carcinoma (HCC) has emerged as one of the most common malignancies worldwide and is characterized by poor prognosis and high mortality. Although surgical resection is considered as the best choice for long-term control of this disease, most advanced HCC patients do not meet the criteria for surgical resection. Moreover, other single-modality treatments, such as chemotherapy, external radiotherapy, and radio frequency ablation, show limited efficacy. Thus, there is a pressing need for multimodal therapies to improve therapeutic responses and prolong the survival of unresectable HCC patients. Recently, continuing efforts led to the thriving combination of chemotherapy and radiotherapy, ultimately achieving optimal and synergetic treatment of unresectable HCC. However, chemoresistance and nonselective cytotoxicity result in low therapeutic efficacy and severe side effects, while radioresistance and the limitations of the irradiative dosages lead to failure to deracinate the hypoxic HCC foci. Moreover, such a synergetic treatment remains unsatisfactory, because radiotherapy and anticancer drugs do not simultaneously affect the same HCC regions. Therefore, it is highly desired to employ an efficient radiosensitizer to enhance the HCC‘s response to radiotherapy, by applying a targeted strategy for exerting maximal synergistic chemoradiotherapeutic effects while with minimal side effects.
Hence, Wen-fei Dong’s group in SIBET designed Janus-structured gold-mesoporous silica nanoparticles (GJNPs) using a modified sol-gel method for integrating chemotherapy, radiotherapy, and in vivo computed tomography (CT) imaging into a single system (Scheme 1). In this system, the folic acid (FA) on the surface of mesoporous silica was modified for HCC targeting, and the subsequent controlled release of the anticancer agent doxorubicin (DOX) was dependent upon acidic stimuli from the tumor microenvironment. The targeted delivery, selective cellular internalization, and pH-controlled drug release of FA-GSJNs-DOX NPs were assessed using hepatoma cell lines and normal liver cell lines with different FA expression levels. In vitro and in vivo experiments were performed to investigate the chemoradiotherapeutic effects and safety of the FA-GSJNs-DOX. Additionally, the CT imaging ability of this Janus nanoplatform was demonstrated in vivo. These results revealed that the combination of chemotherapy and radiotherapy induced synergistic anticancer effects in vitro and exhibited remarkable inhibition of tumor growth in vivo along with significantly reduced systematic toxicity. Additionally, the Janus NPs acted as targeted computed tomography (CT)-imaging agents for HCC diagnosis. Based on the better performance of Janus NPs in targeted chemoradiotherapy and CT imaging, this new nanoplatform designed with dual functionalities might be a promising platform for achieving efficient and safe HCC theranostics. This research has been published in ACS Nano 11, 12, 12732-1274,(SCI, IF= 13.709)https://pubs.acs.org/doi/10.1021/acsnano.7b07486。
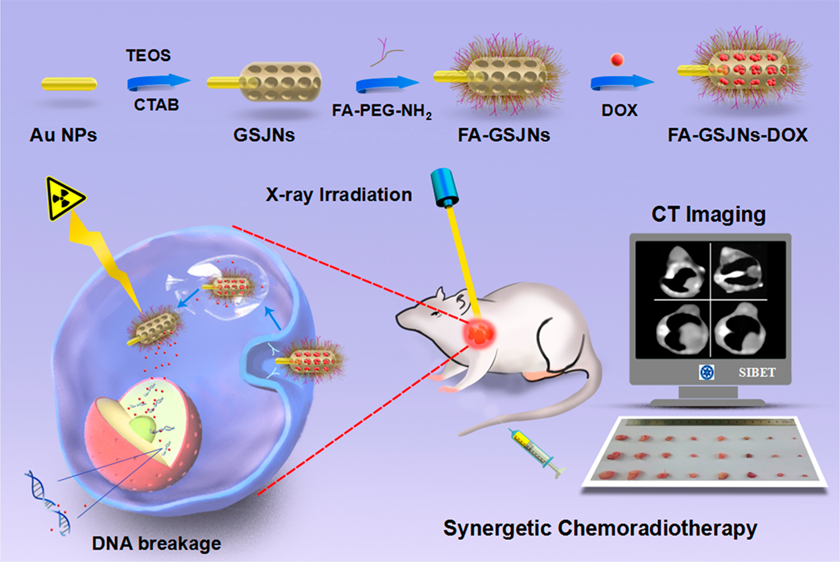
Scheme 1. Janus nanosystem might be a promising platform for achieving efficient and safe HCC theranostics.
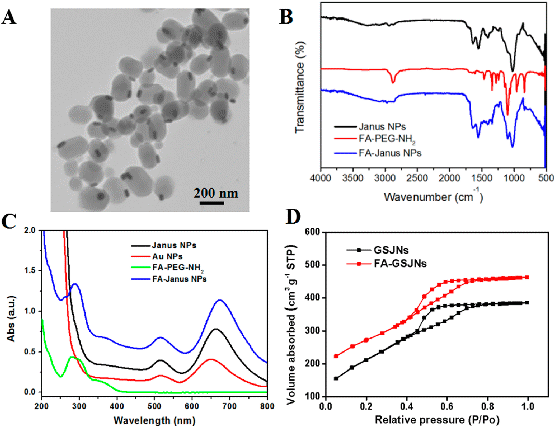
Figure 1. Characterization of FA-GSJNs. TEM images (A), FTIR spectra (B), UV-vis extinction spectra (C), and N2 adsorption-desorption isotherms (D) of FA-GSJNs. These results demonstrated that FA-GSJNs possessed uniform morphology and excellent mesoporous properties.
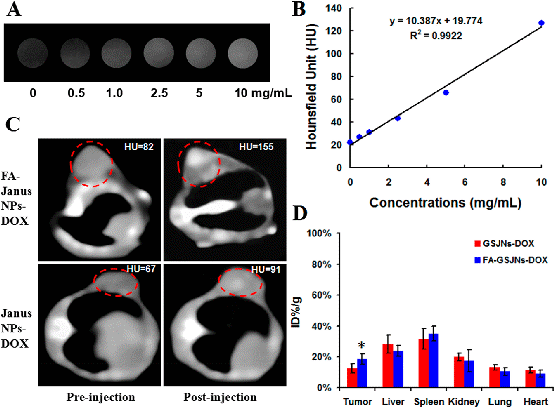
Figure 2. In vivo CT imaging and biodistribution. (A) CT images of distilled water and FA-GSJNs-DOX as a function of concentration. (B) CT attenuation (HU) of FA-GSJNs-DOX at various concentrations (0.5-10 mg/mL). (C) In vivo CT images of SMMC-7721 tumor-bearing nude mice at 24 h post-injection with FA-GSJNs-DOX or GSJNs-DOX. (D) Quantitative ICP-OES analysis of FA-GSJNs-DOX or GSJNs-DOX in organs and tumors at 24 h post-injection. Data represent the mean ± SD (n = 6). *P < 0.05 vs the GSJNs-DOX group. These results demonstrated the superb CT image efficacy of FA-GSJNs
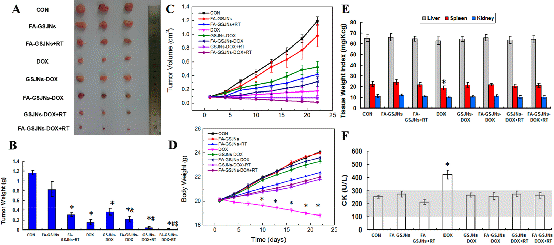
Figure 3. In vivo chemoradiotherapy effect. Tumor photographs (A), weight (B), volume (C), body weight (D), CK levels (E), and weight indexes of the liver, spleen, and kidneys (F) from mice in each group over 23 days. Data represent the mean ± SD (n = 6). These results demonstrated the high-efficiently synergistic therapeutic effect of FA-GSJNs-DOX.
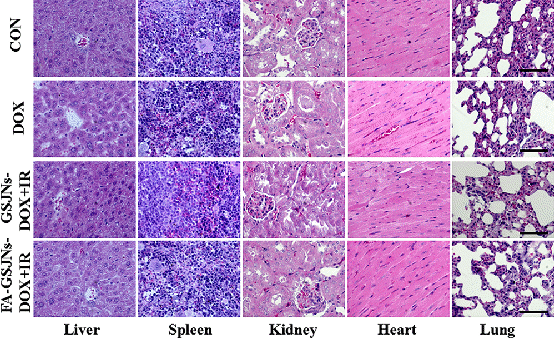
Figure 4. Effect of radiochemotherapy on the histopathology of organs from SMMC-7721 tumor-bearing mice from each group. These results indicated the excellent biosafety of the synergetic chemoradiotherapy based on FA-GSJNs-DOX.