Gastric cancer is a common malignant tumor that originates from the gastric mucosal epithelium. Being insidious and non-specific, the early-stage symptoms of the cancer are similar to that of chronic diseases such as gastritis and gastric ulcer, and are therefore ignored easily. As a result, 80 - 90% of gastric cancer patients have been in the advanced stage when they are first diagnosed. Surgery, with poor prognosis and a 5-year survival rate of only 30%, is still the main treatment for advanced gastric cancer (AGC) at present.
In recent years, neoadjuvant chemotherapy (NACT) has helped improve the prognosis of patients with AGC, and gained increasing popularity among surgeons and patients. However, about 30% of patients with AGC cannot benefit from NACT, but suffer from the risk of disease progression, additional physical damage, and high treatment costs. Although postoperative histopathology is the gold standard for evaluating the NACT efficacy, it cannot assist in optimizing cancer treatment plans. Therefore, accurate identification of AGC patients with NACT resistance before treatment is crucial.
Not long ago, the research team led by GAO Xin from Suzhou Institute of Biomedical Engineering and Technology (SIBET) of the Chinese Academy of Sciences (CAS), collaborating with Shanxi Provincial Cancer Hospital, proposed an Artificial Intelligence (AI) - based method for predicting the efficacy of NACT for AGC (research findings have been published in eClinicalMedicine, 2022, 101348). On this basis, the team recently has proposed, based on intelligent technology for computing medical images, a novel efficacy prediction method that can solve the problem of identifying AGC patients with NACT resistance. As shown in Figure 1, the team built an end-to-end NACT efficacy prediction model for AGC by: using the ResNet-50 neural network architecture to automatically mine high-dimensional features from tumor images, fusing spatial features of the tumor by using a multi-channel image input strategy, and utilizing the boundary information of tumor to guide the network focus on the lesion area. This study adopted CT images of 633 patients with AGC from three hospitals for model training and validation.
The results showed that the proposed model has high prediction accuracy (greater than 0.75 on the internal and external test sets) and strong generalization, making it the best end-to-end NACT response prediction model in prior studies. In addition, to further visualize the interpretability of the model, the team quantified the correspondence between tumor images and chemotherapy resistance using a visual approach. As shown in Figure 2, the activation region of the tumor model in the CT image is not uniform, providing a reference for identifying the implicit association between tumor heterogeneity and chemotherapy resistance.
This study was funded by the National Natural Science Foundation of China, and its findings entitled “Deep learning predicts resistance to neoadjuvant chemotherapy for locally advanced gastric cancer: a multicenter study” have been published in Gastric Cancer (IF: 7.708).
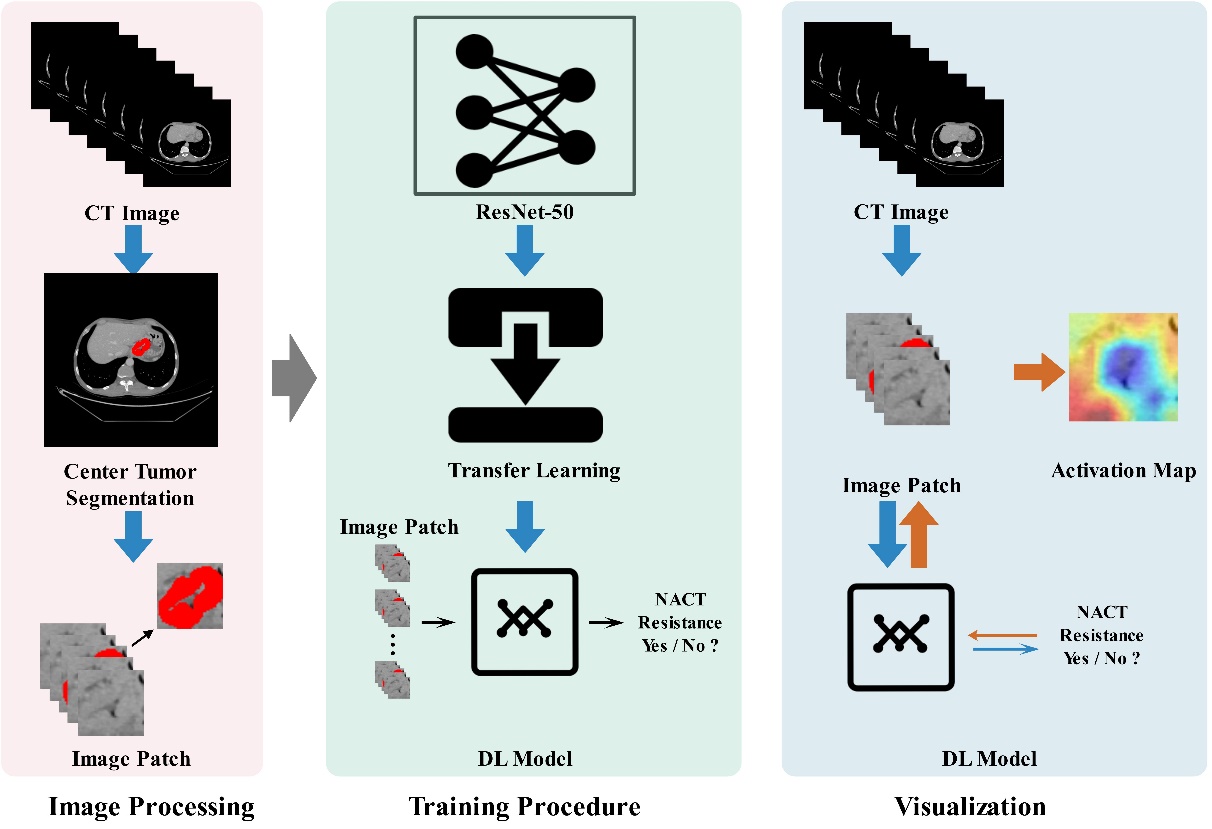
Figure 1. Process for predicting the NACT efficacy in AGC. (Image by SIBET)
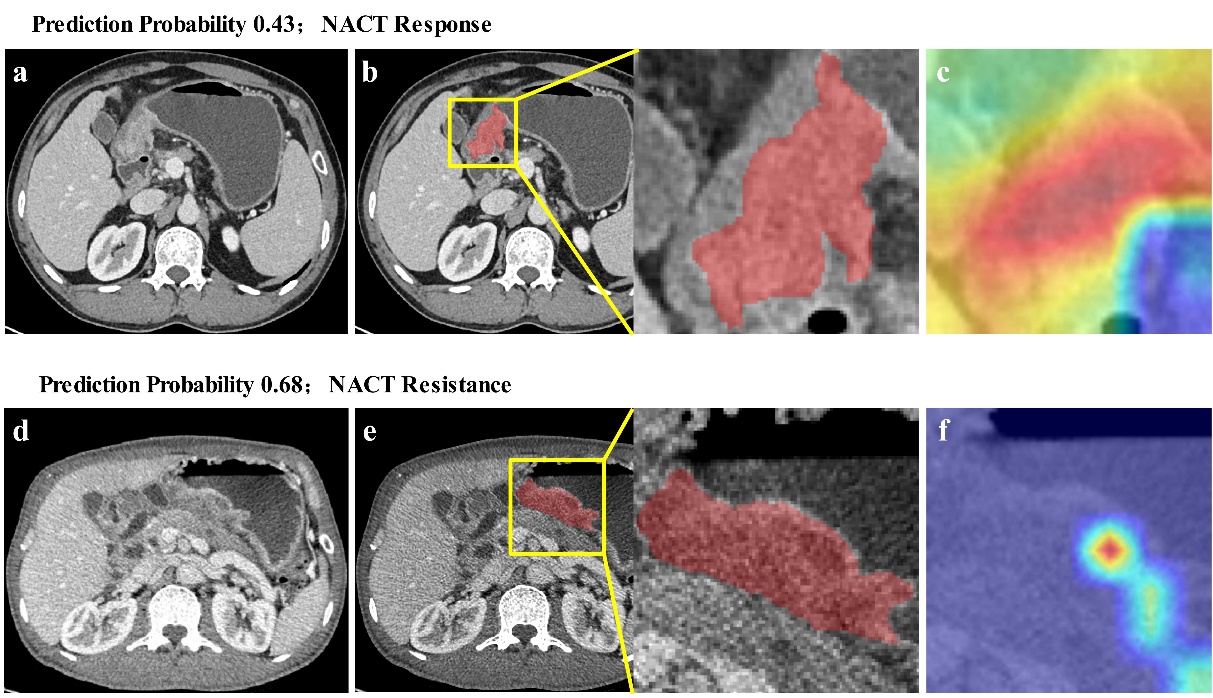
Figure 2. Visualization figures of the prediction model for the NACT efficacy in AGC. (Image by SIBET)
Contact
XIAO Xintong
Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences (http://www.sibet.cas.cn/)
Phone: 86-512-69588013
E-mail: xiaoxt@sibet.ac.cn