Inflammatory bowel diseases (IBD), such as Crohn's disease and ulcerative colitis, are typified by their chronic and recurrent manifestation within the gastrointestinal tract, and are recognized as being associated with an elevated risk of developing colitis-associated colorectal cancer (CAC). Nonetheless, the exact etiology of IBD is yet to be fully elucidated. Myelodysplastic syndrome (MDS) is characterized by dysplastic alterations in hematopoietic stem cells, thereby heightening the propensity for the onset of acute myeloid leukemia (AML). Case reports from patients with inflammatory bowel diseases (IBD) have revealed a significant incidence of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), while a substantial prevalence of IBD was also observed within a sizable cohort of patients suffering from MDS. This suggests a close association between IBD and MDS/AML.
In a study published in Nature Immunology on August 5th, researchers at the Suzhou Institute of Biomedical Engineering and Technology (SIBET) of the Chinese Academy of Sciences, together with collaborators at Shandong University and Tongji University, discovered the critical role of NPM1 in the progression of IBD. It should be noted that NPM1 is also a top driver gene in MDS and AML, which explains the high concurrent rate between IBD and MDS/AML.
The research team found that NPM1 was reduced in IBD patients, and Npm1+/- mice exhibited an enhanced susceptibility to experimentally induced acute colitis and CAC than their littermate controls. Further analysis indicated that Npm1 deficiency compromised production of IL-22 in group 3 innate lymphoid cells (ILC3s). Mice lacking Npm1 in ILC3s exhibited reduced production of IL-22 and an accelerated progression of colitis.
In the mechanism investigation of NPM1 in regulating ILC3 function under IBD mice, the team found that NPM1 could bind with p65 and regulate the transcription of mitochondrial transcription factor A (Tfam), thus promoting the mitochondrial function of ILC3 and supporting the activation of ILC3 under intestinal inflammation (Figure 1). More importantly, the researchers demonstrated that overexpression of Npm1 in mice enhanced ILC3 function and reduced the severity of IBD.
“Our findings uncovered the protective role of NPM1 in maintaining gut homeostasis and implied that a reduction in NPM1 activity is a critical factor bridging the connection between IBD and MDS/AML,” said SUN Minxuan from SIBET, the leading researcher of this study.
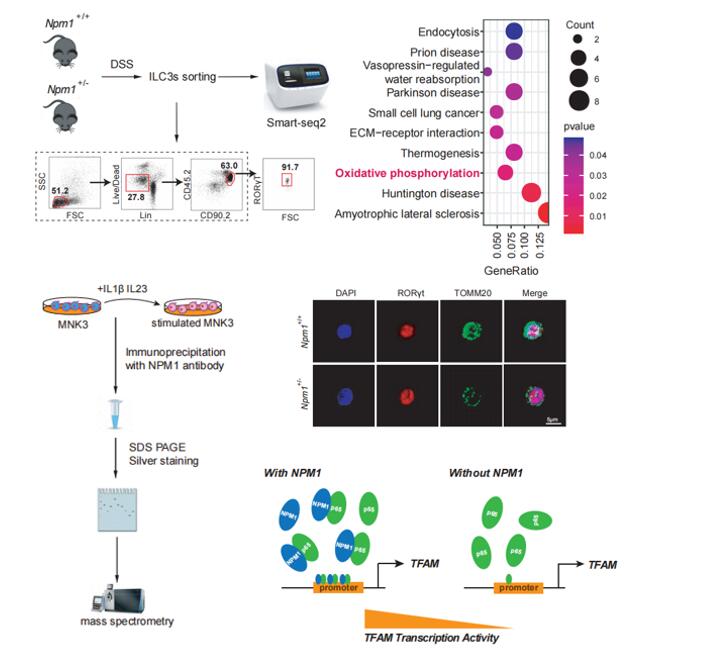
Figure1.NPM1is essential for maintaining mitochondrial OXPHOS and biogenesis inILC3s. (Image by SIBET)
Contact
XIAO Xintong
Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences (http://www.sibet.cas.cn/)
Phone: 86-512-69588013
E-mail: xiaoxt@sibet.ac.cn