|
|
|
Cell Analysis instruments Research Center |
 |
Text Size:
A
A
A |
|
1. Introduction By conducting researches on the detection, identification, and statistical analysis of individual characteristics of cells and microspheres including structure, morphology, specific maker, and fluorescent probes coding, method of cell surface molecules analysis and intracellular antigen detection, DNA content detection, soluble protein molecular analysis are developed to achieve high-sensitivity innovative analysis based on statistical methods, to develop related reagents, instruments and standards to meet the requirements of stem cell medical treatment, disease prognosis tracking, personalized medicine and other needs. Flow cytometry
|
Principle:Cells, microspheres and other particles labeled by fluorescent are suspended in fluid and injected into flow chamber. With hydrodynamic focusing, the sample particles are arranged in a single row and discharged at high speed and perpendicularly intersect with the laser. Particles pass through the detection zone to produce scattering lights. Scattering lights are selected by filters, received by photodetectors and converted into electrical signals. The electrical signals are stored, processed, displayed, analyzed with software. |
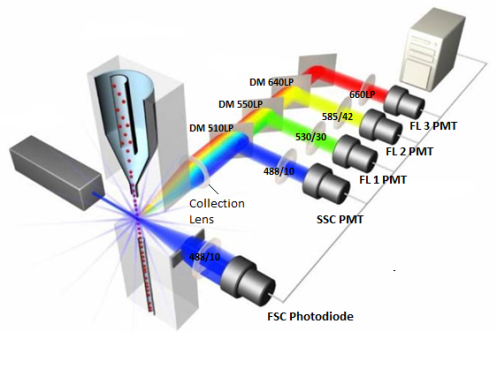
Principle of flow cytometer
-
The forward scattered light intensity is bound up with the size of the measured particles;
-
the side scattered light intensity is related to the interior complexity of the particles
-
the fluorescence wavelength is associated with the surface antigen of the particles |
|
|
|
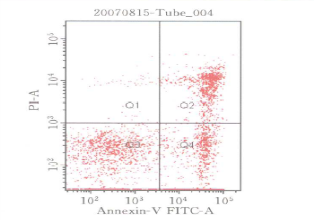
Applications:Flow cytomtery is widely used in immunology, hematology, oncology, cell biology, cytogenetics, biochemistry, clinical medicine and basic medical research, such as:
Clinical
-
AIDS Surveillance (FDA Gold Standard)
-
transplantation, radiotherapy and chemotherapy status evaluation
-
white blood cell antigen type
Cell biology
-
Stem cell counting and activity analysis
-
cell culture, apoptosis cycle detection
-
surface molecular detection
Food, environmental monitoring
-
algae (environmental pollution monitoring, marine ecological detection)
-
Yeast (Biotechnology Optimization)
Biological agents
|
|
Data and applications | -
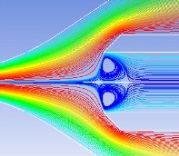
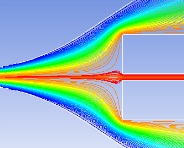
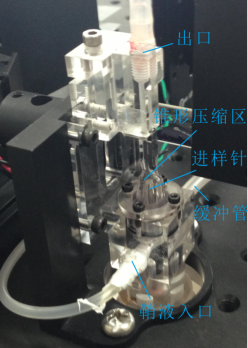
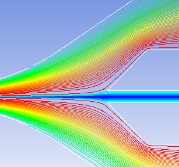
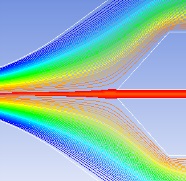
Hydrodynamic focusing technique
|
Sample streamline must be stable and the particles are required to pass through the detection zone one by one. Several factors are considered:
-
sample, sheath flow rate
-
syringe structure
-
syringe position
-
sheath injection method
-
flow pulsation |
|
|
|
|
|
|
|
Flow chamber | -
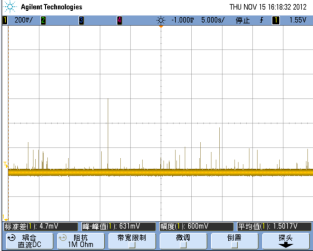
Weak fluorescence detection technology
|
Flow cytometry is targeted for cells or microspheres whose diameters are generally 2-20 microns; The flow analysis is conducted at high flow rate, which means that the single particle passes through the detecting zone in less than 10 microseconds. These two factors cause the signal to be very weak. |

|
|
|
Fluorescence detector and microsphere fluorescence detection results | -
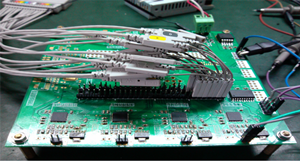
Multi-channel high-speed synchronous signal acquisition technology
-
multi-channel high-speed data processing chip to achieve 16bit, 25MHz sampling rate
-
FPGA hardware logic algorithm to identify the signal peak and area and compress data |
|
|
|
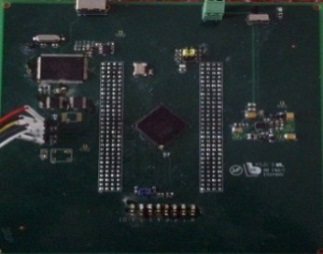
Electronic circuit | -
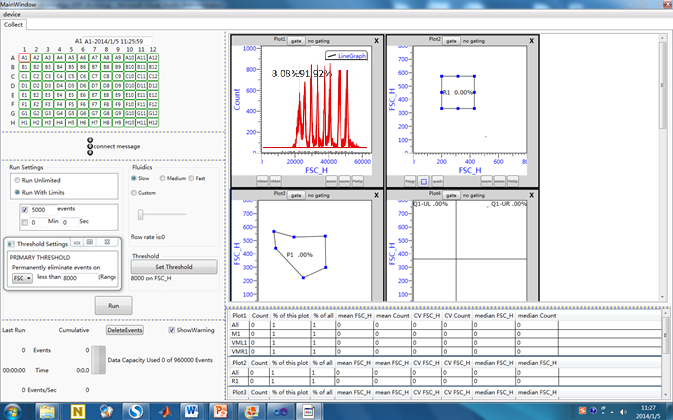
data analysis and software
-
Hardware control include: laser control, flow rate control, detector gain control, acquisition channel settings, trigger settings, data acquisition event control, sampling action control, cleaning action control.
-
Data display and analysis include: histogram, scatter, compensation, set the door, data statistics. |
|
|
Software | Prototype
|

|
Parameters
-
Laser Excitation: 488 nm
-
Emission Detection:
FL1:525/45nm (e.g. FITC)
FL2:592/43nm (e.g. PE)
FL3:692/40nm (e.g. PE-Cy5.5)
FL4:785/62nm (e.g. PE-Cy7)
High: 60μl/min
Medium: 35μl/min
Low: 10μl/min
Properties
-
Fluorescence Sensitivity:PE ≤ 100MESF; FITC ≤ 200MESF
-
Fluorescence Linearity:≥0.98
-
Forward scatter resolution:≤1μm
-
Fluorescence resolution (CV of forward and Fluorescence scatter):≤2%
-
Forward and side scatter resolution:Scatter performance is optimized for resolving lymphocytes, monocytes, and granulocytes.
-
Sampling rate: 10,000 events per second with 18 parameters
-
Carryover: Less than or equal to 1.0%
|
|
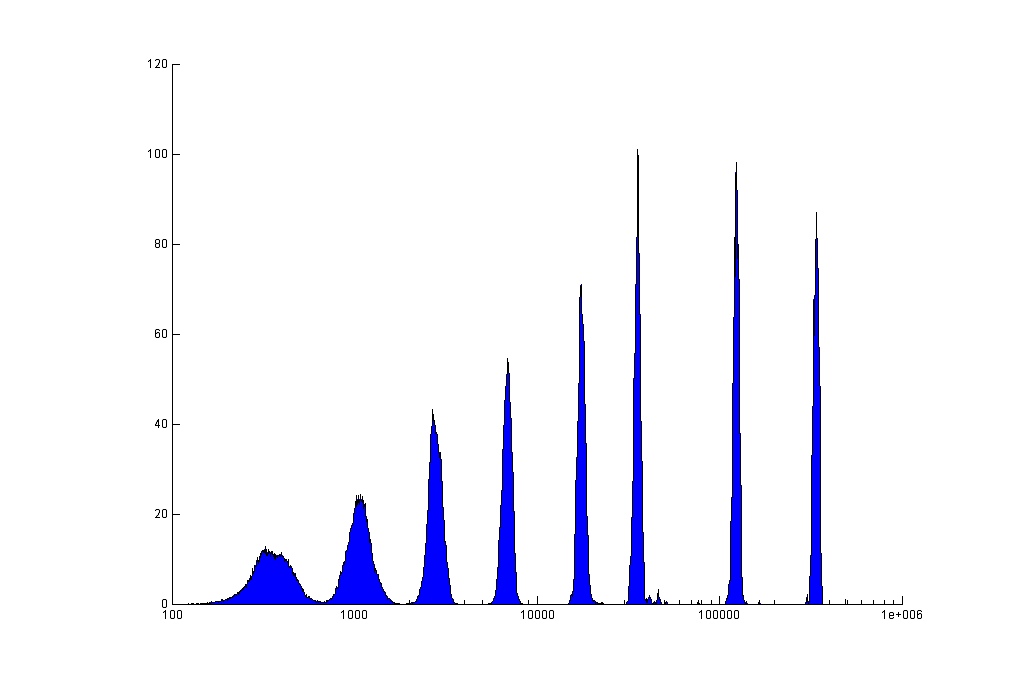
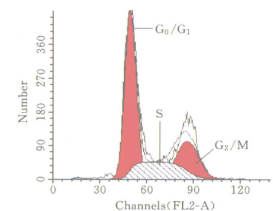
Testing results of Rainbow sphere |
-
10 times the dynamic response range of traditional cytometers; 16bit multi-channel simultaneous sampling A/D signal conversion
-
constant volume without valve fluid delivery system and microfluidic nozzle technology to achieve sample quantification, stable microflow output; buffer technology to weaken the flow rate changes caused by pulsation in flow
-
long-life all-solid-state lasers to achieve bio-fluorescent probe excitation. The integrated optics reduces the effect of laser spot drift and attenuates noise due to spot jitter. | |
|